Sarepta Refuses FDA Request to Halt Elevidys After Three Deaths
Sarepta Therapeutics Inc. has refused a request from the U.S. Food and Drug Administration to halt all shipments of its gene therapy Elevidys after three patient deaths, Bloomberg reported this weekend.
The FDA disclosed that two teenage boys with Duchenne muscular dystrophy, both unable to walk, died recently from acute liver failure after receiving Elevidys. In addition, a 51-year-old participant in a trial of a different Sarepta gene therapy targeting limb-girdle muscular dystrophy died last month, also of acute liver failure.
Bloomberg writes that following these events, FDA officials met with Sarepta and asked the company to voluntarily pause shipments of Elevidys, which accounts for more than half the company’s product revenue. “The company refused to do so,” the agency stated.
Sarepta, in its own statement Friday, defended its decision to continue distribution “based on our comprehensive scientific interpretation of the data, which shows no new or changed safety signals” in patients who are still ambulatory.
In June, the company had already suspended Elevidys shipments to non-ambulatory patients. According to Sarepta, boys who can still walk represent about 85% of those treated with the therapy since its launch.
FDA Commissioner Marty Makary said in an interview Friday the agency is reviewing whether Elevidys should remain on the market.
The most recent death involved a gene therapy using the same viral delivery platform as Elevidys, suggesting potential broader safety implications. “We think the risks of the FDA removing the drug fully from the market are now greatly amplified,” Baird analyst Brian Skorney wrote in a Friday note.
On Friday, at around 1:00 p.m. ET, Reuters reported—citing a source familiar with the matter—that the U.S. Food and Drug Administration would request Sarepta to halt all shipments of Elevidys. By 1:30 p.m. ET, the news has been reported by Bloomberg.
The company had confirmed a third patient death linked to its gene therapy programs, this time involving a 51-year-old man who died in June from acute liver failure after receiving the investigational therapy SRP-9004 for limb-girdle muscular dystrophy (LGMD).
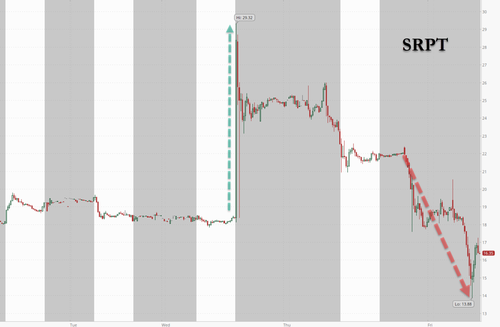
The company confirmed the death to BioSpace and it was widely reported on Friday morning. Sarepta shares were lower by about 25% heading into the cash open and ultimately closed down more than 30%.
Like Elevidys—Sarepta’s approved Duchenne muscular dystrophy gene therapy—SRP-9004 uses an adeno-associated virus (AAV) vector, which the company has previously associated with fatal liver complications.
The death, which went unmentioned during Sarepta’s corporate restructuring update earlier last week, has drawn scrutiny from analysts.
„We think the LGMD patient death could amplify patient hesitancy to use commercial Elevidys and increase investor distrust since the company did not disclose the event on its call,” William Blair noted Friday.
Tyler Durden
Mon, 07/21/2025 – 05:45