
Sarepta Price Target Slashed To $0 At H.C. Wainwright As Stock Plunge Continues
Shares of Sarepta dropped as much as 11% in premarket trading and remain down about 6% after the cash open this morning.
The decline follows H.C. Wainwright’s reiteration of its Sell rating and a drastic cut in its price target—from $10 to $0—after the FDA requested Sarepta voluntarily withdraw its ELEVIDYS gene therapy from the market. Sarepta has reportedly declined to comply.
The FDA’s request follows a third liver-related death linked to the company’s gene therapy programs—two associated with ELEVIDYS and one from a separate LGMD subtype trial.
Sarepta shares have already plunged more than 90% over the past year, now trading at $14.08, down from a 52-week high of $150.48. According to Bloomberg, the stock now holds 7 Buy, 16 Hold, and 3 Sell ratings.
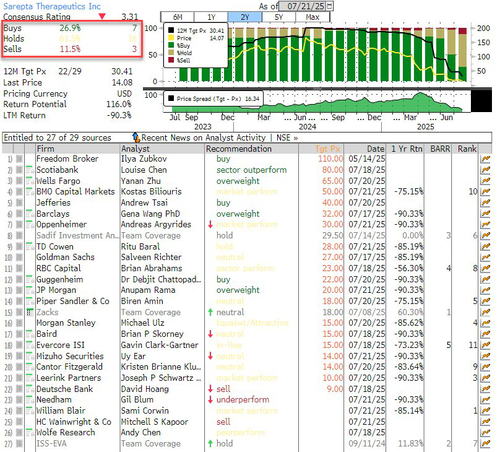
H.C. Wainwright raised concerns over Sarepta’s deteriorating commercial outlook, noting that its non-ELEVIDYS franchise is expected to decline, with 2025 revenue guidance of $900 million, down 6% year-over-year. Its LGMD2E asset, while still in development, addresses a very small patient population—estimated at just 250 cases annually.
Financially, Sarepta faces mounting pressure: it holds $1.1 billion in convertible debt due in 2027, against $850 million in cash.
Sarepta has refused a request from the U.S. Food and Drug Administration to halt all shipments of its gene therapy Elevidys after three patient deaths, we reported this weekend.
The FDA disclosed that two teenage boys with Duchenne muscular dystrophy, both unable to walk, died recently from acute liver failure after receiving Elevidys. In addition, a 51-year-old participant in a trial of a different Sarepta gene therapy targeting limb-girdle muscular dystrophy died last month, also of acute liver failure.
Bloomberg writes that following these events, FDA officials met with Sarepta and asked the company to voluntarily pause shipments of Elevidys, which accounts for more than half the company’s product revenue. “The company refused to do so,” the agency stated.
The stock indicates that the prevailing sentiment seems to be that the FDA may pull Sarepta’s drug off the market…
Tyler Durden
Mon, 07/21/2025 – 10:25











